In this brief workshop conducted by the SQR Regulatory Intelligence and Strategy Unit team you will understand the concepts, application of intelligence activities and regulatory strategy of medical devices and the benefits for your business!
SQR Consulting will hold the following Virtual Workshop:
Regulatory intelligence and strategy for medical devices – Key instruments for the competitive advantage of your business
Date: 12/01/2020
Hours: 9: 00-12: 00
Content
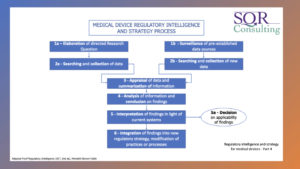
– Overview of regulatory intelligence and strategy for medical devices
– Concepts of regulatory intelligence for medical devices
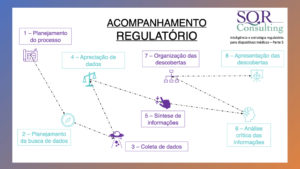
– Regulatory monitoring
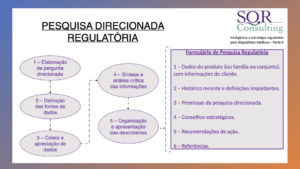
– Targeted regulatory research
– Concepts of regulatory strategy for medical devices
– Device regulatory strategy plan
– How to apply and benefit from the concepts intelligence and strategy of medical devices in your business
Registration by email: inteligenciaregulatoria@sqrconsulting.com.br
The event will be free and will be in Portuguese.
The target audience is medical device manufacturers and importers. Some other organizations, such as government and regulators, may also participate.
In the coming days, we will publish a series of posts showing the concepts, in preparation for the Workshop.




Be First to Comment